The new Pfizer infectious-disease healthy-living may not see Emergency Use Authorization for another month as health officials continue to highlight the promising effects it may bring.
Dr. anthony-fauci” target=”_blank”>Anthony Fauci<, which someone would take within 48 hours of showing symptoms and continue to take for three to five days.
FAUCI: US ‘DEFINITELY’ SAW VARIANTS COMING, BUT OMICRON MUTATIONS ‘UNPRECEDENTED’
Initial trial data indicates that the pill is up to 90% effective at preventing serious illness and death, which has prompted Pfizer and officials to seek Emergency Use Authorization (EUA). Under the authorization, hospitals could administer the pill for treatment in extreme cases.
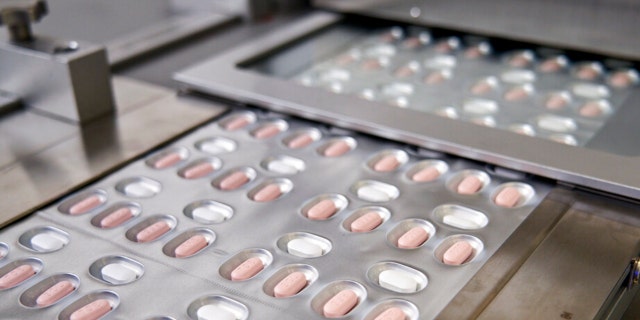
FILE – This undated image provided by Pfizer in November 2021 shows the company’s COVID-19 pills. On Tuesday, Dec. 14, 2021, Pfizer said that its experimental COVID-19 pill is effective against the omicron variant and maintained its promising early performance against the virus in final testing. (Pfizer via AP, File)
((Pfizer via AP, File))
“If you look at that data, the data are really quite impressive,” Fauci said on ABC’s “This Week.” “If you get an antiviral, that up to 90 percent will prevent you from going from clinically recognizable infection to blocking. You’re getting to the hospital or dying in a 90 percent chance if you get treated within the first three days of the onset of symptoms.”
OMICRON FORCES WAVE OF CLOSURES NATIONWIDE AS BIDEN WARNS WINTER OF ‘DEATH’ ENCROACHING
“That is big deal,” he stressed, but the timeline for approval may prove frustrating.

FILE – Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, speaks during the daily briefing at the White House in Washington, Wednesday, Dec. 1, 2021. U.S. health officials said Sunday, Dec. 5 that while the omicron variant of the coronavirus is rapidly spreading throughout the country, early indications suggest it may be less dangerous than delta, which continues to drive a surge of hospitalizations. President Joe Biden’s chief medial adviser, Dr. Anthony Fauci, told CNN’s "State of the Union" that scientists need more information before drawing conclusion’s about omicron’s severity. (AP Photo/Susan Walsh, File)
The Pfizer COVID-19 vaccine concluded its Phase III trials and released data on Nov. 18, 2020. The Food and Drug Administration (FDA) took around one month to review the data and pass the EUA on Dec. 11.
NFL REDUCES TESTING FOR ASYMPTOMATIC VACCINATED PLAYERS
The vaccine did not receive full approval until Aug. 23, 2021, which presents a very grim expectation for the pill’s availability.

FDA dismissed approach to halve Moderna vaccine doses, saying it lacks sufficient evidence and poses a significant public health risk.
(iStock)
That means the FDA would still need three to four weeks to pass the EUA for the Pfizer pill, and Fauci cautioned that it may be months before the public sees widespread availability. He also pointed to the production needs, which also took a month or two for Pfizer to reach mass-production and led to the initial prioritization of vaccinations at the end of last year.
“It’s going to be months,” Fauci explained. “If you look, it’s a very complicated synthetic process to make the drug. It is not something that’s simple.”
CLICK HERE TO GET THE FOX NEWS APP
“So the companies revving up and getting more and more, but we’re not going to see widely available for at least a few months,” he added.
 Iktodaypk Latest international news, sport and comment
Iktodaypk Latest international news, sport and comment